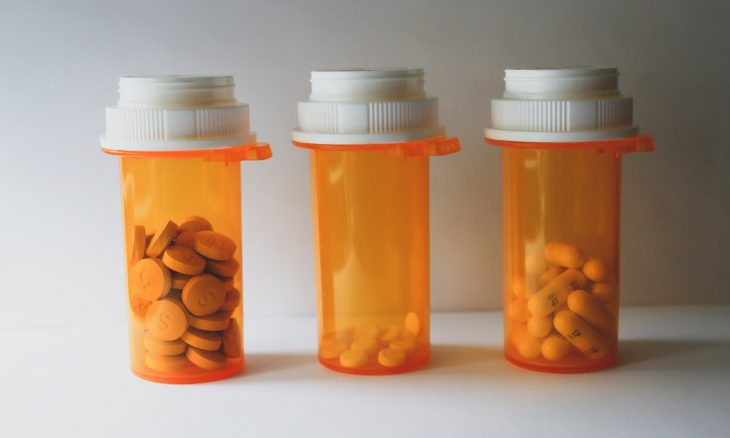
New labeling rules follow evidence linking extended opioid use to serious health dangers, including addiction and overdose.
The U.S. Food and Drug Administration (FDA) has mandated updated safety labels for all prescription opioid pain medications to more clearly reflect the risks associated with long-term use. The decision follows a May advisory committee meeting that reviewed studies showing increased dangers of misuse, dependence, and both fatal and non-fatal overdoses in patients using opioids over extended periods.
The FDA’s review concluded that existing labels lacked adequate warnings and failed to reflect the absence of well-controlled studies supporting long-term use. The new labels will include stronger warnings on addiction risks, dose-related harm, and the dangers of abruptly discontinuing medication, along with guidance for when long-acting opioids should be considered.
“The death of almost one million Americans during the opioid epidemic has been one of the cardinal failures of the public health establishment,” said FDA Commissioner Marty Makary. “This long-overdue labeling change is only part of what needs to be done — we also need to modernize our approval processes and post-market monitoring so that nothing like this ever happens again.”
In addition to labeling changes, the FDA is now requiring a new clinical trial to more rigorously study the long-term effects of opioids. Drug manufacturers must submit the updated labels within 30 days.
As the Lord Leads, Pray with Us…
- For Secretary Kennedy to seek the Lord as he oversees the review of federal health regulations.
- For Commissioner Makary to receive God’s guidance as he heads the Food and Drug Administration.
Sources: Department of Health and Human Services