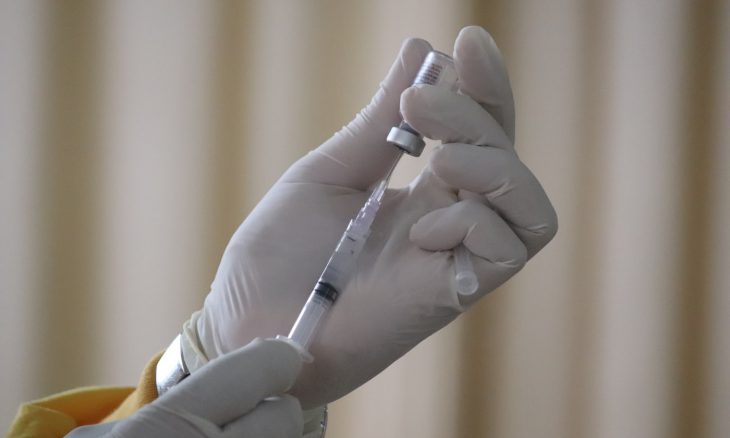
Approval paused for evaluation of myocarditis in males under 30.
The Food and Drug Administration is reportedly delaying approval of Moderna’s COVID-19 authorization for teens to study the possibility of heart inflammation. Instances of myocarditis in men under 30 are the reason for the pause as researchers have found that both the Moderna and Pfizer vaccines could potentially cause the condition.
The FDA has slowed the approval process after Denmark, Sweden, Finland, and Norway issued recommendations against the Moderna vaccine in those who are under 30. Children and youth are not included in the categories of those who are at high-risk from the virus.
As the Lord Leads, Pray with Us…
- For officials in the FDA to determine any dangers to teens prior to authorizing COVID inoculations.
- For the president’s administration to seek God’s guidance in protecting the health of the public.
Sources: JustTheNews, NY Post