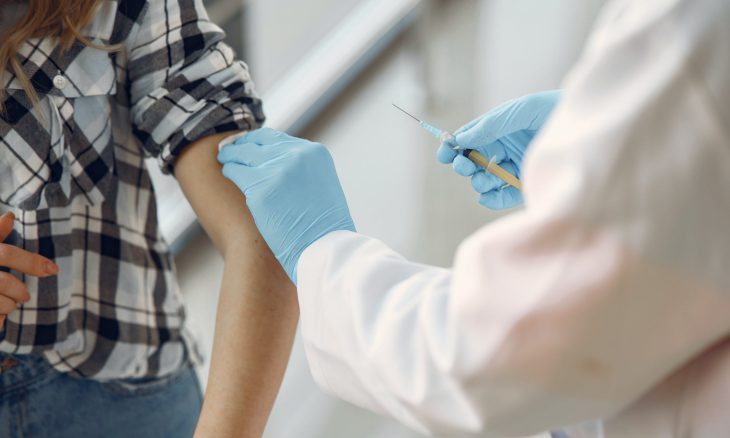
“KidCOVE” study involves Moderna and NIH.
Moderna announced Tuesday it has begun clinical trials of its two-dose vaccine in children from six months to 11 years in both the U.S. and Canada.
“This pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger population,” Moderna’s CEO said.
The study known as “KidCOVE” will enroll 6,750 children, with each child given both doses of the vaccine 28 days apart—the same schedule as for adults. It will be conducted in cooperation with the National Institutes of Health, the federal government’s largest medical research agency.
Moderna has already launched clinical trials for children 12 to 17, but it has not indicated when the varied studies will conclude.
Pfizer has also begun trials to determine vaccine safety in children. Johnson & Johnson expects to have a vaccine safe enough for children under 18 by September.
As the Lord Leads, Pray with Us…
- For the safety of the children who will be participating in the clinical trials for the coronavirus vaccine.
- For the continued decline of the coronavirus and reopening of the nation.
- For NIH to ensure the safety of all vaccines before they implement them.
Sources: Washington Examiner, CBS News