More testing could lead to getting local economies reopened.
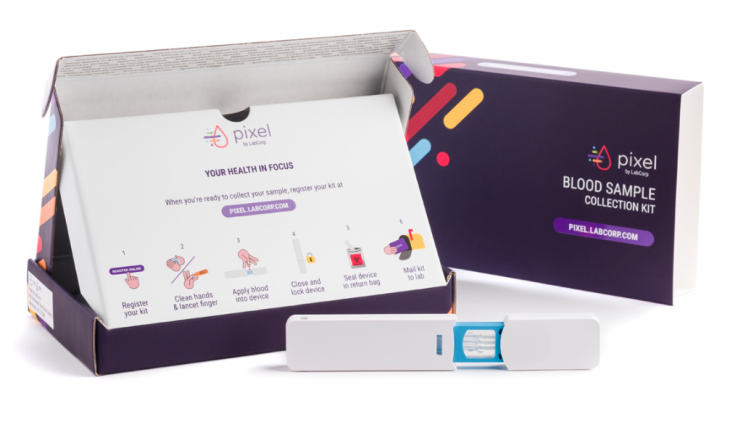
The Food and Drug Administration has approved the first at-home COVID-19 virus sample-collection kits under an Emergency Use Authorization. It would allow people to collect a sample at home using LabCorp’s coronavirus test, known as “Pixel.”
Sample collection materials are included in the kit, including nasal swabs. Once complete, users can mail their samples to LabCorp for testing.
“Throughout this pandemic we have been facilitating test development to ensure patients access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options,” FDA Commissioner Stephen Hahn said in a statement.
Referring to Pixel, LabCorp’s website said, “We’re excited to begin making kits available to healthcare workers and first responders.” They will receive priority access to the kits because “initial quantities of kits are limited.” Individuals must complete a short eligibility survey then, if eligible, purchase their kit that will be sent to them through FedEx, collect their sample and send it back to the lab for the testing. Results will be made available online.
As the Lord Leads, Pray with Us…
- For the at-home sample collection kits to be expanded so that individuals other than the healthcare workers and first responders will be able to take advantage of them.
- About the need for greater testing in order that state and regional economies can reopen.
- For all who continue to suffer through the COVID-19 virus.
- With thanksgiving for the more than 73,000 people in the U.S. who have recovered from the virus.
Sources: Washington Examiner, LabCorp
RECENT PRAYER UPDATES